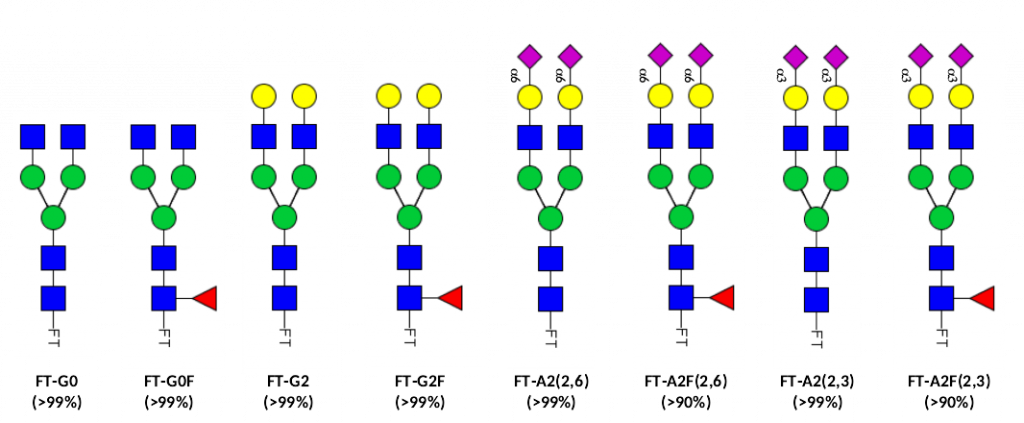
N-glycan standards compatible with GlycoWorks™ RapiFluor-MS™
Glycan standards – compatible with GlycoWorks™ RapiFluor-MS™ are synthetic fluorescently labeled N-glycans, produced by Asparia Glycomics as standards for N-glycan analysis workflows. They are available as individual standards in high purity (90-99%).
Format: 100 pmol standard vials (up to 10 injections), freeze-dried.
Improved Glycan Profiling using Glycan Standards – compatible with GlycoWorks™ RapiFluor-MS™
- Enhanced ionization efficacy
- Fluorescent group for detection at Ex 265 and Em 425 nm
- Suitable for UHPLC, HPLC, ESI-MS, LC-ESI-MS and LC-FLD systems
- Improve identification of very low abundance glycans and N-glycan structures in complex mixtures
- Characterized by UHPLC-FLD and MS, high purity (90-99%)
- Compatible with GlycoWorks™ RapiFluor-MS™ technology
Applications
- N-glycan identification by either mass spectrometry or fluorescent detection
- Identification of α(2,3) or α(2,6) sialic acid linkages in bis-sialylated biantennary N-glycans
- Identification of relative glycosylation in biopharmaceutical development and quality control
- Development of fast, robust LC-FLD methods
- De-convolution of co-eluting peaks in LC-FLD/MS
- Quality control for instrument, method, and column performance
Application Note:
Instructions for Glycan Standards – compatible with GlycoWorks™ RapiFluor-MS™
- Upon arrival store Glycan Standards – compatible with GlycoWorks™ RapiFluor-MS™ vials at -20 °C. For long-term storage, store at -80 °C.
- Sold as a lyophilizate, recommended solubilization reconstitution is in a mixture of 30 μL of deionized water and 70 μL of acetonitrile added directly to the vial for a total volume of 100 μL.
- The recommended injection volume is 8-10 μL for up to 10 injections per vial.
Stored at -80 °C Glycan Standards – compatible with GlycoWorks™ RapiFluor-MS™standards are stable for >3 years.
-
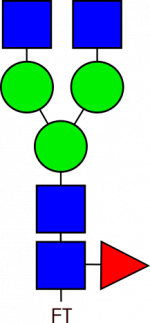 Glycan Standard Biantennary G0F – compatible with GlycoWorks™ RapiFluor-MS™
Glycan Standard Biantennary G0F – compatible with GlycoWorks™ RapiFluor-MS™ -
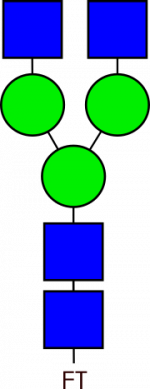 Glycan Standard Biantennary G0 – compatible with GlycoWorks™ RapiFluor-MS™
Glycan Standard Biantennary G0 – compatible with GlycoWorks™ RapiFluor-MS™ -
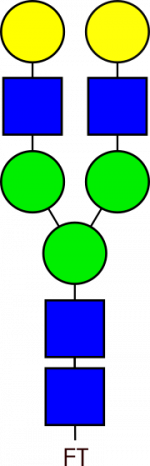 Glycan Standard Biantennary G2 – compatible with GlycoWorks™ RapiFluor-MS™
Glycan Standard Biantennary G2 – compatible with GlycoWorks™ RapiFluor-MS™ -
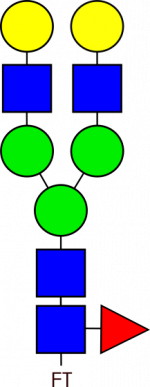 Glycan Standard Biantennary G2F – compatible with GlycoWorks™ RapiFluor-MS™
Glycan Standard Biantennary G2F – compatible with GlycoWorks™ RapiFluor-MS™ -
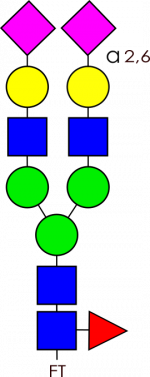 Glycan Standard Biantennary A2F(2,6) – compatible with GlycoWorks™ RapiFluor-MS™
Glycan Standard Biantennary A2F(2,6) – compatible with GlycoWorks™ RapiFluor-MS™ -
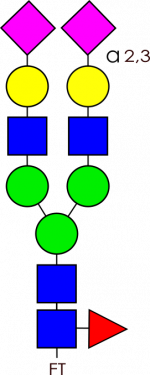 Glycan Standard Biantennary A2F(2,3) – compatible with GlycoWorks™ RapiFluor-MS™
Glycan Standard Biantennary A2F(2,3) – compatible with GlycoWorks™ RapiFluor-MS™ -
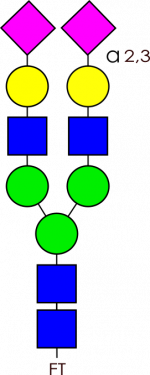 Glycan Standard Biantennary A2(2,3) – compatible with GlycoWorks™ RapiFluor-MS™
Glycan Standard Biantennary A2(2,3) – compatible with GlycoWorks™ RapiFluor-MS™
13C CarboQuant Glycan Standards – by Asparia Glycomics
Absolute Quantification for unprecedented precision and enhanced robustness of mass spectrometry-based glycan analysis.
Our CarboQuant Technology based on internal SIL glycan standards for absolute quantification reduces analysis time from days to minutes delivering the highest precision to your diagnostics pipelines.
Quantify individual glycan biomarkers or full glycan profiles by mass spectrometry with unprecedented precision and fidelity using our proprietary standards and kits.

Improved Glycan Profiling using 13C CarboQuant Standards
- Accelerate your glycan-related analysis by up to 95%
- No need for fluorescent labeling and chromatography
- Reduces the risk of human errors
- Streamlined workflow increases efficiency: by eliminating labeling and chromatography steps, labs save precious time and costly reagents
- High accuracy: Use of internal standards maximizes speed, accuracy, and robustness of glycan analysis
- High sensitivity: limit of quantification (LOQ) 0.5pmol, limit of detection (LOD) 0.1 pmol
- Method linearity: linearity over 3 orders of magnitude
-
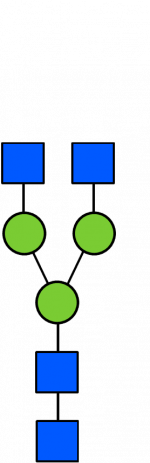 Glycan Standard 13C Biantennary G0
Glycan Standard 13C Biantennary G0 -
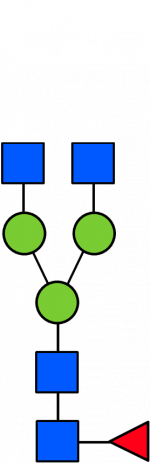 Glycan Standard 13C Biantennary G0F
Glycan Standard 13C Biantennary G0F -
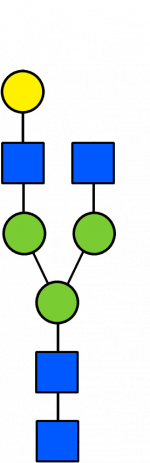 Glycan Standard 13C Biantennary G1(3)
Glycan Standard 13C Biantennary G1(3) -
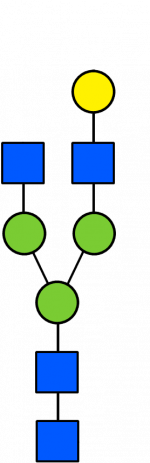 Glycan Standard 13C Biantennary G1(6)
Glycan Standard 13C Biantennary G1(6) -
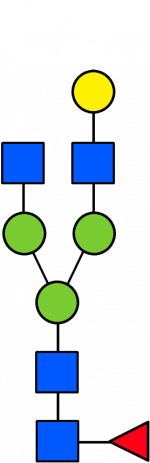 Glycan Standard 13C Biantennary G1(6)F
Glycan Standard 13C Biantennary G1(6)F -
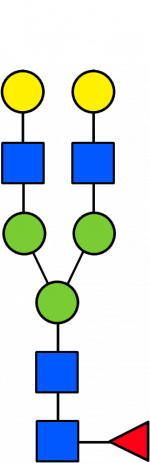 Glycan Standard 13C Biantennary G2F
Glycan Standard 13C Biantennary G2F -
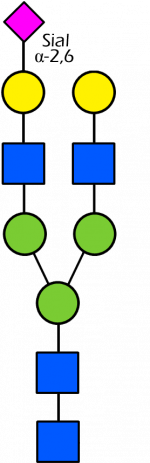 Glycan Standard 13C Biantennary G2A1(3) (Sial α-2,6)
Glycan Standard 13C Biantennary G2A1(3) (Sial α-2,6) -
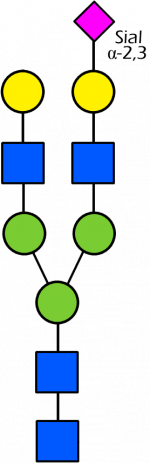 Glycan Standard 13C Biantennary G2A1(6) (Sial α-2,3)
Glycan Standard 13C Biantennary G2A1(6) (Sial α-2,3) -
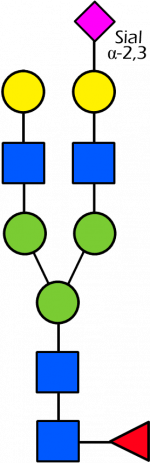 Glycan Standard 13C Biantennary G2A1(6)F (Sial α-2,3)
Glycan Standard 13C Biantennary G2A1(6)F (Sial α-2,3) -
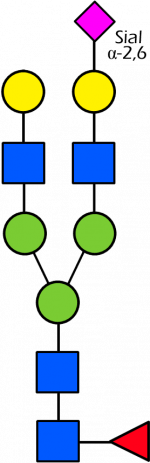 Glycan Standard 13C Biantennary G2A1(6)F (Sial α-2,6)
Glycan Standard 13C Biantennary G2A1(6)F (Sial α-2,6) -
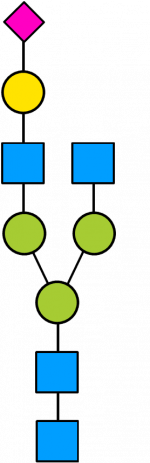 Glycan Standard 13C Biantennary A1(3) (Sial α-2,3)
Glycan Standard 13C Biantennary A1(3) (Sial α-2,3) -
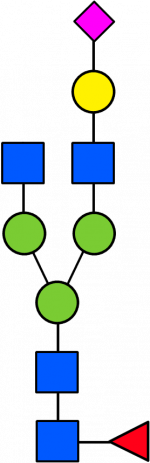 Glycan Standard 13C Biantennary A1(6)F (Sial α-2,3)
Glycan Standard 13C Biantennary A1(6)F (Sial α-2,3) -
 Glycan Standard 13C Biantennary A2 2x(Sial α-2,3)
Glycan Standard 13C Biantennary A2 2x(Sial α-2,3) -
 Glycan Standard 13C Biantennary A2F 2x(Sial α-2,3)
Glycan Standard 13C Biantennary A2F 2x(Sial α-2,3) -
 Glycan Standard 13C Biantennary A2F 2x(Sial α-2,6)
Glycan Standard 13C Biantennary A2F 2x(Sial α-2,6) -
 Glycan Standard 13C Biantennary bA2F 2x(Sial α-2,6)
Glycan Standard 13C Biantennary bA2F 2x(Sial α-2,6) -
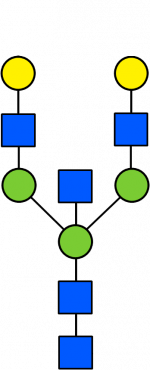 Glycan Standard 13C Biantennary bG2
Glycan Standard 13C Biantennary bG2 -
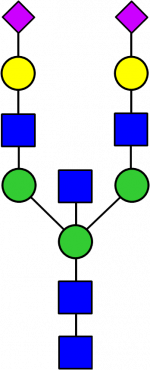 Glycan Standard 13C Biantennary bA2 2x(Sial α-2,6)
Glycan Standard 13C Biantennary bA2 2x(Sial α-2,6) -
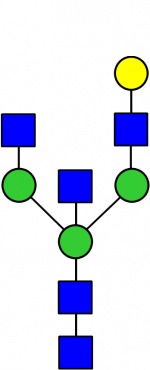 Glycan Standard 13C Biantennary bG1(6)
Glycan Standard 13C Biantennary bG1(6) -
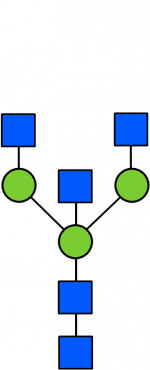 Glycan Standard 13C Biantennary bG0
Glycan Standard 13C Biantennary bG0 -
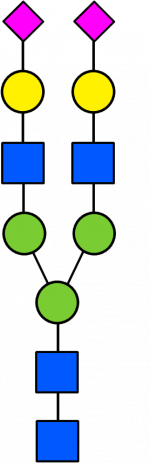 Glycan Standard 13C Biantennary A2 2x(Sial α-2,6)
Glycan Standard 13C Biantennary A2 2x(Sial α-2,6) -
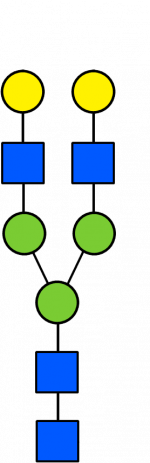 Glycan Standard 13C Biantennary G2
Glycan Standard 13C Biantennary G2 -
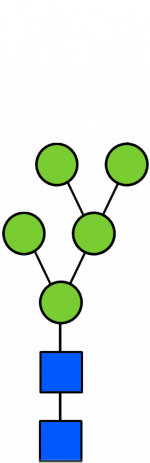 Glycan Standard 13C Paucimannose Man5
Glycan Standard 13C Paucimannose Man5 -
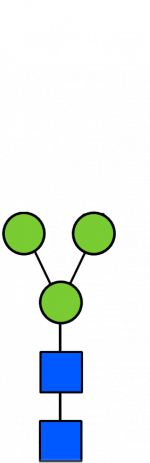 Glycan Standard 13C Paucimannose Man3
Glycan Standard 13C Paucimannose Man3 -
 Glycan Standard 13C truncated MGn(3)F
Glycan Standard 13C truncated MGn(3)F
Customized kits
Feel free to choose from a constantly extended range of SIL-standards to include in a kit customized to your analytical application.
Applications
Glycobiology
- Rapid absolute glycan quantification
- Measure of glycan recovery in sample preparation procedures
- Internal standards for the quantitative glycan profiling by MALDI-Tof MS employing a wide variety of samples: protein, glycopeptides, oligosaccharides, and mAbs.
- Internal standards as reference points for increasing method robustness
Biopharmaceutical Development
- Rapid high-throughput glycan analysis in clone selection and process development
- Quality control
- Biosimilar optimization
Biomarker Quantification
- Measure glycan levels in serum samples with picomole precision
- Establish cut-off values for glycan biomarkers
- Quantify aberrant glycosylation in cancer samples
- Internal performance calibrants for method transfer and multiple laboratory studies
